联系人:苏先生
手 机:18612706349
电 话:010-57297418
传 真:010-57297418
邮 箱:suylinchcn@163.com
地 址:北京市北京经济技术开
发区荣华中路8号院13
号楼10层1111
邮 编:100176
光合电子传递分段反应的测定
光合电子传递photosynthetic electron transport
光合作用中,受光激发推动的电子从H2O到辅酶Ⅱ(NADP+)的传递过程。光合色素吸收光能后,把能量聚集到反应中心,一种特殊状态的叶绿素a分子,引起电荷分离和光化学反应。一方面将水氧化,放出氧气;另一方面把电子传递给辅酶Ⅱ(NADP+),将它还原成NADPH,其间经过一系列中间(电子)载体。绿色植物中,光合电子传递由两个光反应系统相互配合来完成。一个是吸收远红光的特殊叶绿素a分子,最大吸收峰在700nm处,称为P700。由P700和其他辅助复合物组成的光反应系统,称光系统I(PSI)。

另一个是吸收红光的特殊叶绿素a分子,其吸收峰在680nm处,称为P680。由P680和其他辅助复合物组成的光反应系统,称光系统Ⅱ(PSⅡ)。两个光系统之间由细胞色素b6-f和铁硫蛋白组成的复合物连接。在外加人工电子受体和供体的情况,光合链上的电子传递可分段进行。电子传递可偶联氧的产生(水的光解)或消耗(O2作电子受体)。因此可用氧电极测定加入不同电子供体和受体之后氧气的变化反映光合电子传递的分段反应。
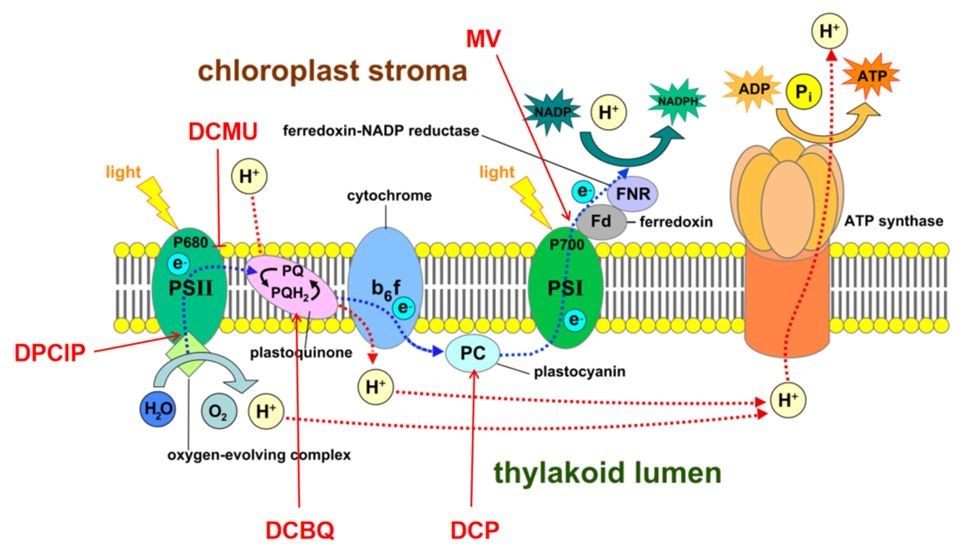
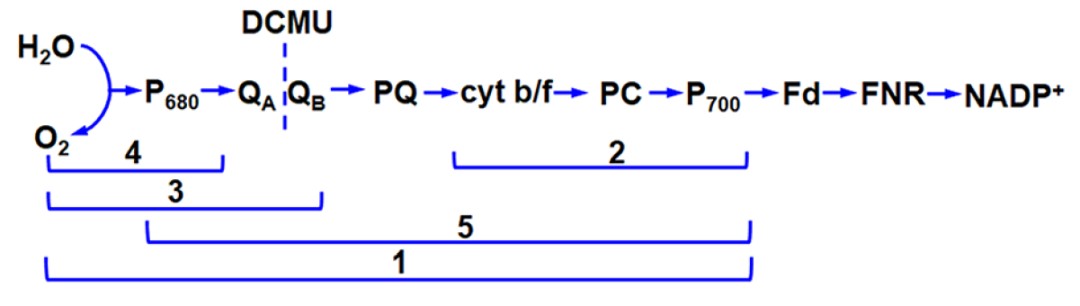
上图是光合电子传递模式图。红色字体:DPC、DCBQ、DCP和MV代表人工电子受体和供体。DCMU是电子从PSII传递到PQ库的抑制剂。1、从水到甲基紫精(MV)的电子传递(耗O2反应:每传递4个电子消耗1个O2,终产物为H2O2)2、从二氯酚靛酚(DCPIP)到甲基紫精的电子传递(耗O2反应:每传递一个电子消耗1个O2)。3、从水到对二氯苯醌DCBQ的电子传递(放氧反应:每传递4个电子释放1个O2)。4、从二苯卡巴肼(DPC)到甲基紫精的电子传递(耗氧反应:传递1个电子消耗1个O2)。下表是测定光合电子传递分段反应所需的体系和试剂:
|
基质(低渗介质) |
分段反应过程 |
反应体系 |
电子传递类型 |
备注 |
|
50mmol/L Tricine-KOH 50mmol/L KCl 5mmol/L MgCl2 PH 7.6 20-50µg chl/ml 叶绿体 |
完整的 电子传递过程 |
50µmol/L MV 5mmol/L NH4Cl 2mmol/L NaN3 |
H2O→MV |
耗氧反应 |
|
PSI活性 |
50µmol/L MV 5mmol/L NH4Cl 2mmol/L NaN3 50µmol/L DCMU 2mmol/L DCPIP |
DCPIP→MV |
耗氧反应 |
|
|
PSII活性 |
5mmol/L NH4Cl 4mmol/L K3Fe(CN)6 1mmol/LDCBQ |
H2O→DCBQ |
放氧反应 |
|
|
PSI和PSII活性 |
5mmol/L NH4Cl 2mmol/L NaN3 0.5mmol/L DPC |
DCP→MV |
耗氧反应 |
注意!!!由于实验样品和条件的差异,测定光合电子传递分段反应所使用的人工电子供体、受体以及反应体系会有所不同,具体试剂和体系请根据实际情况选定。

文献目录:
o Baker, N. R., & Leech, R. M. (1977).Development of photosystem I and photosystem II activities in leaves oflight-grown maize (Zea mays). PlantPhysiology, 60(4), 640-644.
o Baldi, P., Muthuchelian, K., & La Porta, N.(2012). Leaf plasticity to light intensity in Italian cypress (Cupressussempervirens L.): Adaptability of a Mediterranean conifer cultivated in theAlps. Journal of Photochemistry andPhotobiology B: Biology, 117,61-69.
o Chen, S., Zhou, F., Yin, C., Strasser, R. J.,Yang, C., & Qiang, S. (2011). Application of fast chlorophyll afluorescence kinetics to probe action target of 3-acetyl-5-isopropyltetramicacid. Environmental and ExperimentalBotany, 73, 31-41.
o Chen, Y. E., Su, Y. Q., Mao, H. T., Nan, W.,Zhu, F., Yuan, M., ... & Yuan, S. (2018). Terrestrial plants evolvehighly-assembled photosystem complexes in adaptation to light shifts. Frontiers in plant science, 9, 1811.
o Chen, Z., Jiang, H. B., Gao, K., & Qiu, B.S. (2019). Acclimation to low ultraviolet‐B radiation increases photosystem Iabundance and cyclic electron transfer with enhanced photosynthesis and growthin the cyanobacterium Nostoc sphaeroides. Environmentalmicrobiology.
o Ciornii, D., Kölsch, A., Zouni, A., &Lisdat, F. (2019). Exploiting new ways for a more efficient orientation andwiring of PSI to electrodes: A fullerene C70 approach. Electrochimica Acta, 299,531-539.
o Dall'Osto, L., Ünlü, C., Cazzaniga, S., &van Amerongen, H. (2014). Disturbed excitation energy transfer in Arabidopsisthaliana mutants lacking minor antenna complexes of photosystem II. Biochimica et Biophysica Acta(BBA)-Bioenergetics, 1837(12),1981-1988.
o Ding, S., Lei, M., Lu, Q., Zhang, A., Yin, Y.,Wen, X., ... & Lu, C. (2012). Enhanced sensitivity and characterization ofphotosystem II in transgenic tobacco plants with decreased chloroplastglutathione reductase under chilling stress. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1817(11), 1979-1991.
o Dobrikova, A. G., & Apostolova, E. L.(2015). Damage and protection of the photosynthetic apparatus from UV-Bradiation. II. Effect of quercetin at different pH. Journal of plant physiology, 184,98-105.
o Gandini, C., Schmidt, S. B., Husted, S.,Schneider, A., & Leister, D. (2017). The transporter Syn PAM 71 is locatedin the plasma membrane and thylakoids, and mediates manganese tolerance inSynechocystis PCC 6803. New Phytologist,215(1), 256-268.\
o Hakkila, K., Antal, T., Rehman, A. U., Kurkela,J., Wada, H., Vass, I., ... & Tyystjärvi, T. (2014). Oxidative stress andphotoinhibition can be separated in the cyanobacterium Synechocystis sp. PCC6803. Biochimica et Biophysica Acta(BBA)-Bioenergetics, 1837(2),217-225.
o Kiss, É., Knoppová, J., Aznar, G. P., Pilný,J., Yu, J., Halada, P., ... & Komenda, J. (2019). A photosynthesis-specificrubredoxin-like protein is required for efficient association of the D1 and D2proteins during the initial steps of photosystem II assembly. The Plant Cell, 31(9), 2241-2258.
o Kubota, H., Sakurai, I., Katayama, K.,Mizusawa, N., Ohashi, S., Kobayashi, M., ... & Wada, H. (2010).Purification and characterization of photosystem I complex from Synechocystissp. PCC 6803 by expressing histidine-tagged subunits. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1797(1), 98-105.
o Leganes, F., Martinez-Granero, F.,Muñoz-Martín, M. Á., Marco, E., Jorge, A., Carvajal, L., ... &Fernandez-Pinas, F. (2014). Characterization and responses to environmentalcues of a photosynthetic antenna-deficient mutant of the filamentous cyanobacteriumAnabaena sp. PCC 7120. Journal of plantphysiology, 171(11), 915-926.
o Li, Z., Wang, W., Ding, C., Wang, Z., Liao, S.,& Li, C. (2017). Biomimetic electron transport via multiredox shuttles fromphotosystem II to a photoelectrochemical cell for solar water splitting. Energy & Environmental Science, 10(3), 765-771.
o Lidon, F. C., & Ramalho, J. C. (2011).Impact of UV-B irradiation on photosynthetic performance and chloroplastmembrane components in Oryza sativa L. Journalof Photochemistry and Photobiology B: Biology, 104(3), 457-466.
o Lu, C., & Vonshak, A. (1999).Characterization of PSII photochemistry in salt-adapted cells of cyanobacteriumSpirulina platensis. The New Phytologist,141(2), 231-239.
o Madireddi, S. K., Nama, S., Devadasu, E., &Subramanyam, R. (2019). Thylakoid membrane dynamics and state transitions inChlamydomonas reinhardtii under elevated temperature. Photosynthesis research, 139(1-3),215-226.
o Pang, N., Xie, Y., Oung, H. M. O., Sonawane, B.V., Fu, X., Kirchhoff, H., ... & Chen, S. (2019). Regulation andstimulation of photosynthesis of mixotrophically cultured Haematococcuspluvialis by ribose. Algal Research, 39, 101443.
o Peng, X., Deng, X., Tang, X., Tan, T., Zhang,D., Liu, B., & Lin, H. (2019). Involvement of Lhcb6 and Lhcb5 in PhotosynthesisRegulation in Physcomitrella patens Response to Abiotic Stress. International journal of molecular sciences,20(15), 3665.
o Petrova, N., Stoichev, S., Paunov, M.,Todinova, S., Taneva, S. G., & Krumova, S. (2019). Structural organization,thermal stability, and excitation energy utilization of pea thylakoid membranesadapted to low light conditions. ActaPhysiologiae Plantarum, 41(12),188.
o Rehman, A. U., Kodru, S., & Vass, I.(2016). Chloramphenicol mediates superoxide production in photosystem II andenhances its photodamage in isolated membrane particles. Frontiers in Plant Science, 7,479.
o Robinson, S. J., Deroo, C. S., & Yocum, C.F. (1982). Photosynthetic electron transfer in preparations of thecyanobacterium Spirulina platensis. PlantPhysiology, 70(1), 154-161.
o Rozpądek, P., Nosek, M., Domka, A., Ważny, R.,Jędrzejczyk, R., Tokarz, K., ... & Turnau, K. (2019). Acclimation of thephotosynthetic apparatus and alterations in sugar metabolism in response toinoculation with endophytic fungi. Plant,cell & environment, 42(4),1408-1423.
o Sinetova, M. A., Mironov, K. S., Mustardy, L.,Shapiguzov, A., Bachin, D., Allakhverdiev, S. I., & Los, D. A. (2015).Aquaporin-deficient mutant of Synechocystis is sensitive to salt and high-lightstress. Journal of Photochemistry andPhotobiology B: Biology, 152,377-382.
o Thomas, T. D., Dinakar, C., & Puthur, J. T.(2019). Effect of UV-B priming on the abiotic stress tolerance ofstress-sensitive rice seedlings: Priming imprints and cross-tolerance. Plant Physiology and Biochemistry.
o Tibiletti, T., Rehman, A. U., Vass, I., &Funk, C. (2018). The stress-induced SCP/HLIP family of smalllight-harvesting-like proteins (ScpABCDE) protects Photosystem II fromphotoinhibitory damages in the cyanobacterium Synechocystis sp. PCC 6803. Photosynthesis research, 135(1-3), 103-114.
o Tjus, S. E., Møller, B. L., & Scheller, H.V. (1998). Photosystem I is an early target of photoinhibition in barleyilluminated at chilling temperatures. Plant Physiology, 116(2), 755-764.
o Trebst, A. (1972). [14] Measurement of hillreactions and photoreduction. In Methodsin enzymology (Vol. 24, pp. 146-165). Academic Press.
o Trotta, A., Barera, S., Marsano, F., Osella,D., Musso, D., Pagliano, C., ... & Barbato, R. (2018). Isolation andcharacterization of a photosystem II preparation from thylakoid membranes ofthe extreme halophyte Salicornia veneta Pignatti et Lausi. Plant physiology and biochemistry, 132, 356-362.
o Ungerer, J., Lin, P. C., Chen, H. Y., &Pakrasi, H. B. (2018). Adjustments to photosystem stoichiometry and electrontransfer proteins are key to the remarkably fast growth of the cyanobacteriumSynechococcus elongatus UTEX 2973. MBio,9(1), e02327-17.
o Vajravel, S., Kovács, L., Kis, M., Rehman, A.U., Vass, I., Gombos, Z., & Toth, T. N. (2016). β-Carotene influences thephycobilisome antenna of cyanobacterium Synechocystis sp. PCC 6803. Photosynthesis research, 130(1-3), 403-415.
o Wu, W., Ping, W., Wu, H., Li, M., Gu, D., &Xu, Y. (2013). Monogalactosyldiacylglycerol deficiency in tobacco inhibits thecytochrome b6f-mediated intersystem electron transport process and affects thephotostability of the photosystem II apparatus. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1827(6), 709-722.
o Zhang, H., Lv, J., Peng, Y., Zhang, S., An, X.,Xu, H., ... & Zheng, T. (2014). Cell death in a harmful algal bloom causingspecies Alexandrium tamarense upon an algicidal bacterium induction. Applied microbiology and biotechnology, 98(18), 7949-7958.
o Zhang, Y., Ding, S., Lu, Q., Yang, Z., Wen, X.,Zhang, L., & Lu, C. (2011). Characterization of photosystem II intransgenic tobacco plants with decreased iron superoxide dismutase. Biochimica et Biophysica Acta(BBA)-Bioenergetics, 1807(4),391-403.
o Zhong, X., Li, Y., Che, X., Zhang, Z., Li, Y.,Liu, B., ... & Gao, H. (2019). Significant inhibition of photosynthesis andrespiration in leaves of Cucumis sativus L. by oxybenzone, an active ingredientin sunscreen. Chemosphere, 219, 456-462.
o Zienkiewicz, M., Drożak, A., Wasilewska, W.,Bacławska, I., Przedpełska-Wąsowicz, E., & Romanowska, E. (2015). Theshort-term response of Arabidopsis thaliana (C3) and Zea mays (C4) chloroplaststo red and far red light. Planta, 242(6), 1479-1493.
o Zienkiewicz, M., Krupnik, T., Drożak, A.,Wasilewska, W., Golke, A., & Romanowska, E. (2018). Deletion of psbQ’genein Cyanidioschyzon merolae reveals the function of extrinsic PsbQ’in PSII. Plant molecular biology, 96(1-2), 135-149.
暂无信息
